
Horizontal Autoclave BAHZ-810-B
- Sea, Air, Door to Door Shipping
- 1 Year Warranty
- US & European Standards
We offer a variety of horizontal laboratory autoclaves for general purpose sterilization applications. Customers to choose from the range depending upon their application, volume and budgetary requirements. Features are designed specifically for lab based sterilizations used in research laboratories, pharmaceuticals, microbiology labs, food and chemical industries. High performance, reliable results and a longer life makes it an ideal choice.
Specification
Features
Applications
| Capacity | 650 L |
| Chamber Size (Wx H x L) | 672x672x1500 mm |
| External Size (WxHxL) | 1314x1260x1980 mm |
| Max Designed Pressure | Inner chamber - 0.1/0.3 Mpa, Jacket 0.3MPa |
| Working Pressure | 0.23 Mpa |
| Built - in Steam Generator | 0.8 Mpa |
| Designed Temperature | 150 °C |
| Maximum Working Temperature | 134 °C |
| Temperature Control Precision | ± 0.2 °C |
| Consumption | 56kVA |
| Standard Accessories | Trolley, Printer |
| Altitude | ≤ 2000m; could be customised @ 2000-3000m |
| Power Supply | 380 V 50 Hz |
| Package Size | Main Instrument: 1424x1460x2295; Trolley: 1860x640x1245 |
| Gross Weight | Main Instrument: 1440; Trolley: 130 |
Bulk sterilization, Microbiology Lab, Research Institutions, Laboratory, Pharmaceutical, Food industry, Chemical industry
Operating Manual for BAHZ-810-B
1. Brief introduction
2. Technical Parameters and Other Instructions
3. Product composition and performance
3.1 Product composition
3.2 Product property
3.3 The sterilization unit
4. Installation
4.1 Cartridge inspection
4.2 Equipment installation
4.3 Testing of equipment
5. Operation
5.1 Seal door
5.2 Program run and its parameter settings
5.3 Manual manipulation
5.4 System maintenance
5.5 Parameter setting
6. Daily maintenance, repair and operation procedures of the equipment
7. Common Faults and Solutions
7.1 Common fault information
7.2 Alarm code
7.3 Analysis and exclusion of wet packets
7.4 Analysis and exclusion of unqualified sterilization
8. Quality assurance measures for sterile products
9. Detection of Sterilization Effect
10. Packing List
1. Brief introduction
Product name: Biolab brand BAHZ-810-B large pressure steam sterilizer Warning First time using this product, please read this manual carefully!
Warning First time using this product, please read this manual carefully!When appear
 Warning Check the documents to identify the nature of the potential danger and the measures that must be taken.
Warning Check the documents to identify the nature of the potential danger and the measures that must be taken.Large pressure steam sterilizer(Mobile door)Our company is based according to the latest national standards——《Technical requirements of large steam sterilizer- -automatic control type》One of the series of high-grade sterilization equipment developed and produced is a sterilization device that fully meets the requirements of CSSD and GMP specifications. This series of sterilizers can be used for medical treatment, pharmaceutical, scientific research and other units for surgical instruments, dressings, fabrics and other sterilization purposes.
 Warning This sterilizer should not be used for the sterilization of the undesigned use of loads, such as petroleum products and powder items such as petroleum jelly.
Warning This sterilizer should not be used for the sterilization of the undesigned use of loads, such as petroleum products and powder items such as petroleum jelly. Danger It is strictly prohibited to use the equipment to sterilize the liquid sealed with glass bottles or glass ware, because the change of temperature and pressure in the operation process may cause the glass products to burst, thus endangering the equipment or personnel. The elimination of sterilization indoor air of the equipment adopts the so-called pulsation method of multiple vacuum extraction and multiple steam injection, so that the indoor air exclusion amount reaches above 99%, so as to ensure that the temperature uniformity throughout the room can reach 0.5℃, and ensure the reliable sterilization effect.
Danger It is strictly prohibited to use the equipment to sterilize the liquid sealed with glass bottles or glass ware, because the change of temperature and pressure in the operation process may cause the glass products to burst, thus endangering the equipment or personnel. The elimination of sterilization indoor air of the equipment adopts the so-called pulsation method of multiple vacuum extraction and multiple steam injection, so that the indoor air exclusion amount reaches above 99%, so as to ensure that the temperature uniformity throughout the room can reach 0.5℃, and ensure the reliable sterilization effect.Sterilization indoor shell material is Q245R carbon steel, the internal surface by mechanical polishing and electric polishing treatment, not only beautiful, but also greatly enhanced the corrosion resistance ability. The design and production of the sterilization room shall fully comply with the requirements of the GB150 Steel Pressure Vessel and the Safety Technical Supervision Regulations of the Pressure Vessel.
The inner surface of the door plate material is S30408, the outside is Q235A reinforcing reinforcement, and the outside is all coated with insulation material to minimize heat radiation. The lifting motor of the door is used to drive the chain and other drive mechanism; the silicone seal strip is tightly pressed on the door plate, which is not only sealed reliably, but also can greatly reduce the labor intensity of the operator compared with the manual door. The sterilization is double door (i. e., double door), the front and rear door sealing strip can be operated separately, the front door can be opened separately, when the front door is sealed, so that the bacterial door can be effectively isolated from the sterile area and meet the requirements of GMP and hospital CSSD specification.
Using the touch screen as the man-machine control interface, the temperature, pressure, time and other parameters in the operation process of the program can be dynamically displayed dynamically, and it can be printed and used for archiving for future reference.
With the programmable controller (PLC or PC) for program control, with strong function, high reliability, flexible use and other characteristics.
2. Technical Parameters and Other Instructions
1、 Model:BAHZ-810-B;Nominal working pressure :0.12MPa/0.21MPa ;Rated working temperature:121℃/134℃
2、 Set the range of pulsation times:1—99 times;
3、 Set the range of the sterilization time:0—9999s;
4、 Dry time setting range:0—9999s;
5、 Safety valve setting pressure:0.28MPa;
6、 Water source:Tap water pressure 0.1-0.3mpa; Softened water or pure water should be connected to the buffer tank with a volume of no less than 150L
7、 compressed air:0.5MPa—0.7MPa After oil removal, water filtration and dust filtration treatment;
8、 source:Power power supply:AC 380V/50Hz(Power shall be subject to the specific equipment order number) ;control source :AC 220V/50Hz/0.5kW.
9、 Compressed gas source pressure controller:lower limit :0.4MPa。
10、 Product size(mm):
650L:672±2*672±2*1500±5
11、 Product shape and size(mm):
650L:1314±10*1760±10*2050±15
12、 Net weight of products:BAHZ-810-B:1035kg. If installed on the ground or in a pit, the surface should be solid and smooth. If installed on the upper floor, the user should consider whether the floor needs to be reinforced according to the specific situation.
13、 Main sterilization factor and its strength, sterilization principle and microbial killing category: using saturated steam as sterilization factor; sterilization temperature range and load temperature; for steam sterilization, using the principle of heat factor killing microorganisms, the saturated humid steam as the sterilization factor, in the environment of high temperature, high pressure and high humidity, according to the combination of certain pressure and time. This device can kill both bacteria and spores.
14、 Scope and method of use: for sterilization of wet resistant and heat resistant medical devices. Use the method as detailed in the instruction manual.
15、 Note: See the instructions for details.
16、 Service life of the whole machine: 5 years.
17、 operative norm :《GB 8599-2008 Large-scale steam sterilizer technology requires automatic control type》
3. Product composition and performance
3.1 Product composition
The sterilizer consists of sterilization chamber, sealing door, external decoration cover, pipeline system and control system.3.2 Product property
The lower limit of the sterilization temperature range is the sterilization temperature, and the upper limit shall not exceed the sterilization temperature + 3℃.At the maintenance time, the temperature measured at the sterilization chamber reference measurement point, the temperature of any test point in the standard test package, and the corresponding saturated steam temperature calculated on the basis of the sterilization chamber pressure shall meet the following requirements:
——Should be within the sterilization temperature range;
——The difference between the points at the same time shall not exceed 2℃.
For the sterilizers with the sterilization temperatures of 121℃ and 134℃, the maintenance time was 20min and 7min, respectively.
3.3 The sterilization unit
BAHZ-810-B The standard sterilizer is 4.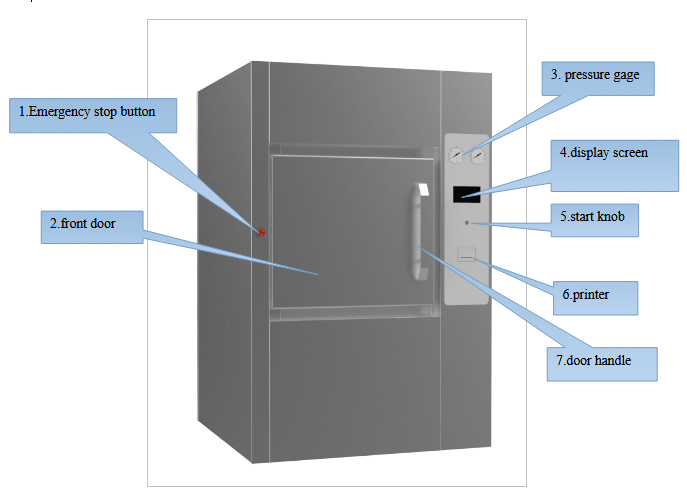
Figure 1
4. Installation
 Warning Whether the correct installation of the sterilization equipment is used will play an important role in its daily performance.
Warning Whether the correct installation of the sterilization equipment is used will play an important role in its daily performance.The equipment is transported after wooden packing and shall be checked for damage before unpacking.
4.1 Cartridge inspection
● After the inventory, carefully check whether the parts of the equipment are in good condition, damaged or lost. If any, make a record well, and contact our company in time.● Carefully check whether the connection or fixings are loose or tightened during the long-distance transportation process. Attach the M22 adjustment bolt to the rack floor first if required.
● After unpacking the equipment, please refer to the equipment packing list to check and record whether the equipment and its random accessories are complete. If you have any questions, please contact our company.
4.2 Equipment installation
Equipment installation shall be under the guidance of professional personnel and under the responsibility of professional construction personnel. Warning Equipment must be installed according to the requirements of the Company, Otherwise, the Company shall not be liable for the consequences caused by the incorrect installation.
Warning Equipment must be installed according to the requirements of the Company, Otherwise, the Company shall not be liable for the consequences caused by the incorrect installation.1)Equipment occupies space occupation and installation requirements
●For the convenience of operation and maintenance, the height of the room should be at least 2.9 meters, and the distance between the left and right sides of the sterilizer and the wall should not be less than 0.5m. When the single sterilizer is installed, the distance between the back end and the wall shall not be less than 0.5m. The distance between the front and rear operation surface of the sterilizer and the opposite wall should be at least 1.5 times of the total length of the equipment, so that the sterilization vehicle can turn freely, push and pull easily.
● Ground: this series of equipment has two ways of ground installation and pit installation. The required surface shall be flat. If installed upstairs, consider whether to reinforce the floor according to the bearing situation of the floor.
● Room ventilation and heat dissipation: According to the latest requirements of CSSD, it is recommended to partition the equipment after being in place to reduce the impact of environmental noise and heat on the working environment. In order to control the temperature of the working environment, a set of ventilation device should be installed in the working room and above the equipment.
● Equipment drainage: The drainage outlet should be slightly larger than the sterilto reduce the back pressure; the drainage pipe should be separately led to the trench, so that part of the gas discharged from the equipment during work will not affect other rooms. The trench is at least 200mm wide and 200mm deep.
 Warning: The setting of the drainage pipe shall prevent the generation of drainage back pressure, otherwise the sterilization or drying effect will be affected due to the poor discharge of condensate water. Drainage pipe materials shall be high temperature resistant (140℃) and anti-aging materials.
Warning: The setting of the drainage pipe shall prevent the generation of drainage back pressure, otherwise the sterilization or drying effect will be affected due to the poor discharge of condensate water. Drainage pipe materials shall be high temperature resistant (140℃) and anti-aging materials.2)Various equipment power equipment requirements
● Steam source(non-electrothermal): it is best to set up a steam water separator on the steam source pipe. The separator can remove the solid particles and condensate carried in the steam delivery process to enable the sterilizer to obtain a high-quality steam source. The steam source pressure is 0.30- -0.50MPa. If the steam source pressure is too high (> 0.5MPa), the pressure relief valve should be added to the transmission pipeline to ensure that the steam source pressure fluctuation does not exceed 10%. Industrial steam source pipe for 1 inch internal thread connection. If the sterilization equipment is far away from the boiler, the diameter of the steam transmission pipe should be increased to reduce the pipe resistance. When double steam, pure steam line is 1 inch joint. Steam conveying pipeline shall be insulated to reduce the loss of steam. In order to observe the steam supply situation timely, the steam source valve and a pressure gauge of 0-1MPa shall be installed on the steam source pipeline into the interlayer or inner chamber.
● Water source: the inlet pipe is a 1 / 2 inch thread pipe. Connect a 0—0.6MPa range pressure gauge and valve to the inlet sterile water pipe to observe and control the water pressure.
 Warning The evaporator should use the water after softening treatment to ensure the long-term normal operation of the evaporator.
Warning The evaporator should use the water after softening treatment to ensure the long-term normal operation of the evaporator.● Compressed gas source: (gas source pressure: 0.5-0.7MPa) compressed gas pipe is 8 (or 6) hose. Compressed air shall be directed by the main road near the sterilizer and fitted with a stop valve, a pressure relief valve, a pressure gauge, and an 8 (or 6) plug-in direct terminal quick change connector.
● If there is no compressed gas source, you can buy a 60 L / minute medical oil-free compressor for a single set of equipment to support the use.
● power supply: the power supply shall be three-phase five-wire system (three-phase fire wire, one zero wire, one ground wire), the user is required to have two power wires, one is for the power power cord of liquid ring vacuum pump, the other is for the control wire. Install the power switch box near the equipment on the rear wall of the sterilizer with a three-phase switch switch (or circuit breaker) and a single-phase switch switch (or circuit breaker). A ground wire must be laid and a control cable with ground number "ground" must be reliably connected to the ground wire.
3)Equipment installation connection
●Move the equipment to the selected location.
● adjustment level
After the carrier equipment is in place, lift the support with the adjustment bolt on the foot of the equipment to suspend the four wheels, place the level instrument on the guide rail of the sterilization chamber, and adjust the above bolts to level the left and right directions of the sterilization device. Then adjust the four bolts on the handling wheel to ensure that the height of the rail matches the height of the sterilization inner guide rail, and lock the fixing nuts (the front two directional wheels should be consistent with the direction of the carrier guide rail). Push the sterilization vehicle from the carrier into or out of the sterilization room, and ensure that the sterilization vehicle should be convenient in and out, and the carrier can push and pull flexibly.
● Water, electricity and steam connection
According to the specific installation location and installation requirements of the equipment, connect water, electricity, steam and compressed gas with the sterilization.
 Warning: The power supply ground cable must be reliably grounded!!The grounding identification is
Warning: The power supply ground cable must be reliably grounded!!The grounding identification is 
Equipment junction box is welded with M5*16 bolts connected to the whole shell and connected to the ground wire.
4.3 Testing of equipment
The mobile gate large pressure steam sterilizer has pre-set up several procedures, during the debugging process, the user can modify the program parameters according to the use requirements. Read chapter 5 for the specific parameter settings《Instructions for the use of equipment》.●Before commissioning, first check whether the electrical partial wiring and socket fall off or become loose, and whether the connection of water inlet, drainage, steam inlet and compressed air inlet is correct.
● Open the water source valve, the steam source valve, and the air compressor valve. Observe whether the pressure indicators meet the use requirements.
1)Check the rotation direction of the vacuum pump
Turn on the power switch to make the touch screen into the manual operation state, start the vacuum pump, and observe whether the rotation direction of the motor fan of the vacuum pump is consistent with the arrow direction marked on the pump body, otherwise any two load lines on the three-phase load switch should be replaced.
2)Check the operation condition of each valve piece
● The pneumatic valve —— is manually operated to open each pneumatic valve one by one to watch whether the red display head of each pneumatic valve extends out.
● Solenoid valve —— in the manual operation state, open each solenoid valve, with a screwdriver gently close to the top of the solenoid valve, should feel a strong magnetic attraction.
3)No-load test of the sterilizer
● Before the program runs, the pressure protection test should be conducted according to the instructions in Chapter 5 "Equipment Use Instructions". If the test is unqualified, it means that the pipeline connected with the inner room is leaking. At this time, please check carefully, eliminate the leakage point, and re-test until the test is qualified, otherwise, the sterilization effect of the equipment will be affected.
● Parameter setting: refer to the basic parameters of the equipment in Chapter 5.
● After parameter setting, normal empty load operation of fabric, instruments, lumen instruments, B-D experiment and other procedures, Please read chapter 5 for the specific procedure《Instructions for the use of equipment》。
4) Load test
The above procedures are carried out under no-load, and the load test should be carried out after passing the no-load test.
In the load test, the loading amount of sterilized articles such as instruments and fabric shall not exceed 80% of the inner chamber volume, and shall be placed on the joist of the sterilization vehicle as required, and the dressing pack and equipment room shall keep a 10mm clearance. The load test must test the sterilization effect of the central center of the package according to the standard requirements.
Conduct B-D test procedures, chemical test test and biological test for the sterilizer according to Chapter 7 to observe whether the test results meet the requirements.
 Warning Allow the sterilizer to modify the program parameters according to the actual situation during debugging.
Warning Allow the sterilizer to modify the program parameters according to the actual situation during debugging.5) Emergency stop switch
The device is equipped with an emergency stop switch on the front and rear end panels. When the equipment program needs to stop the operation, press the switch to stop the program; then press the switch device is in preparation again.
5. Operation
 Warning Non-operators do not touch the control screen to ensure the clarity of the screen!
Warning Non-operators do not touch the control screen to ensure the clarity of the screen!5.1 Seal door
First press chapter 4《 equipment installation 》To prepare the sterilizer, open the general control power supply, steam source, water source, compressed gas source, and open the operation power switch. After the touch screen is powered on, after a period of self-inspection, you can enter the first picture shown in FIG. 1, which timely displays the inner chamber pressure of the equipment and the temperature of the inner chamber, and also performs the screen switching operation.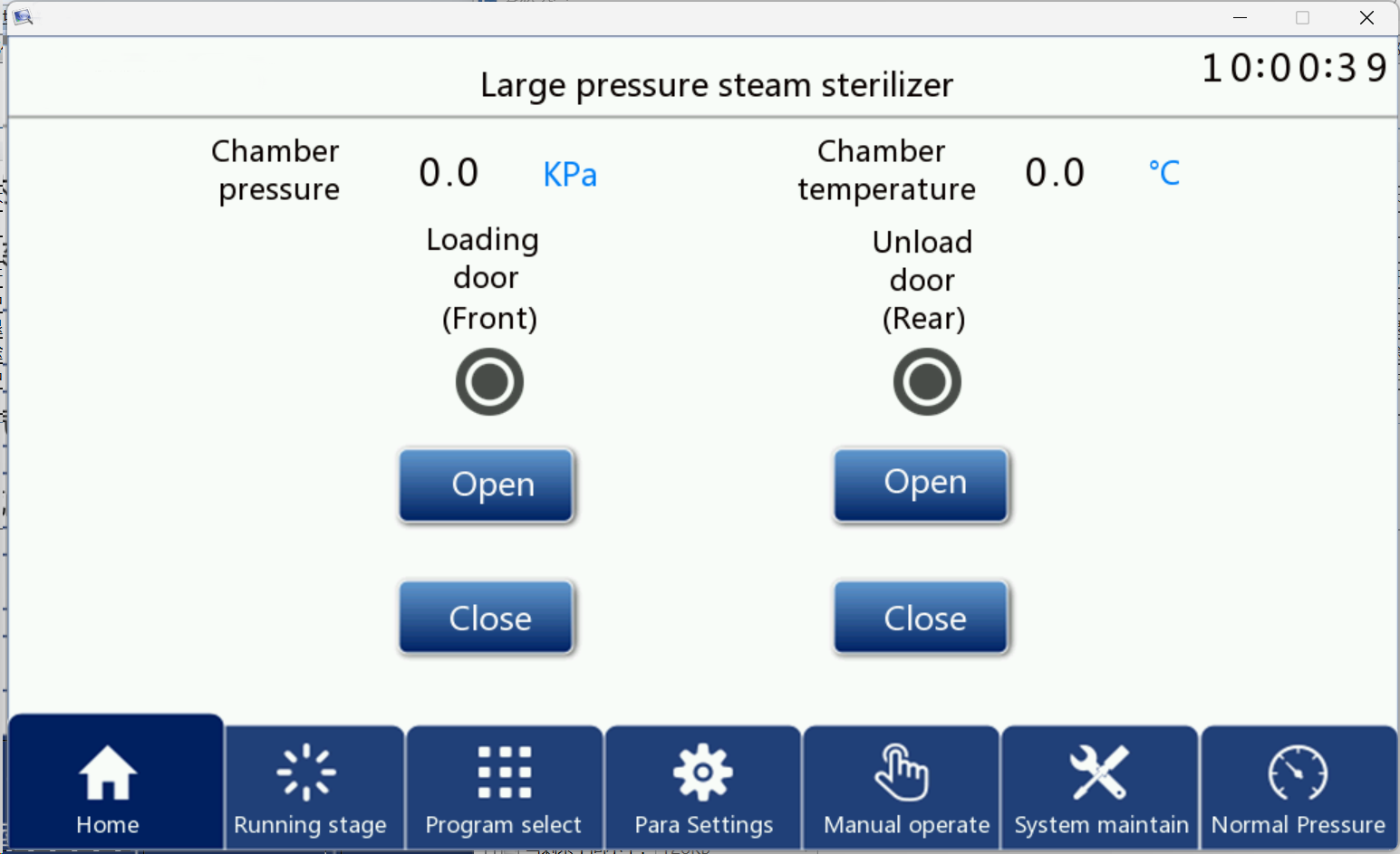
Figure 2
In this screen, there are "front door operation" and "back door operation", two controls relative to the front and rear doors, and the buttons can complete the corresponding functions.
The specific opening and closing operations are as follows:
1)The front door
In the open door opening state, gently close the door to the end frame, when the upper teeth of the door plate enter the sterilization chamber rack, indicating that the front door is closed.
In the manual screen, press the "Close the front Door" button in the front door operation area to close the front door operation. During the front door, press the Close button again to stop the door operation. Make the door completely closed before closing, otherwise the alarm shown in Figure 3 will appear below the main screen. After the door is completely closed, close the door again.

Figure 3
During the closing process, if the operation is abnormal, the program will automatically cancel the closing operation, and the touch screen will display the alarm information, and the alarm sound will prompt the —— buzzer to indicate the corresponding alarm information. The screen is as shown in Figure 4:
This screen will display for 5 seconds, the buzzer will ring for 5 seconds, and the screen will automatically turn off after 5 seconds.

Figure 4
When the vacuum pump starts while closing the front door, while the solenoid valve moves, pull the sealant strip in the sealing groove of the front end box, and the door motor moves. After the door plate reaches the closed position, the door stroke switch is closed, the front door indicator light is on, and close the front door operation is completed. When the door needs to be sealed, the compressed air passes through the solenoid valve into the front-end frame sealing groove, and the sealant strip is pushed out and close to the door plate, to realize the sealing of the door.
2)The front door open
After sterilization, the internal chamber pressure is ± 10KPa. Opening the front door also requires you to switch the touch screen to the door manual screen. After pressing the "open the front door" button on the touch screen, the vacuum pump and the electric ball valve act first, and the compressed air in the sealing groove of the front end box is extracted, and pull the sealant strip back to the sealing groove. After 15 seconds, the door motor moves (if the starting position of the door is not closed, the delay of 15 seconds is unnecessary). In the process of opening the door, if the door motor appears overcurrent protection, indicating that the door meets abnormal resistance in the opening process, the control system will automatically cancel the door opening operation, and prompt the corresponding information, as shown in Figure 4. During the opening of the front door, the front door status indicator light is flashing. When the door panel reaches the opening position, the front door indicator light is out, and the opening front door operation is completed, and the sealed door can be opened.
Open the door operation cannot be performed in the following cases:
a. The internal compartment pressure is not between-10Kpa and 10Kpa. If the door is opened, the alarm screen shown in Figure 5 will appear:

Figure 5
The alarm is over after 5 seconds.
b. The back door is not closed or the back door is not in the normal closed position. The corresponding alarm information is shown in Figure 6 in the alarm screen.

Figure 6
3)Back door gate (double gate)
The backdoor operation is performed by pressing the Close Door button switch on the rear panel. Related information is displayed on the touch screen on the front panel, and the closing process closes the front door along with other related operations.
4)Back door is open (double gate)
The open back door operation is performed by pressing the "open door" button switch on the rear panel of the device. The related information is displayed on the touch screen on the front panel, opening the opening process and opening the front door with other related operations.
5)Door safety interlock device
The equipment is a pressure vessel. In order to ensure the safety of the equipment operators, the equipment design must ensure that the current and back door is not closed; when the indoor pressure is greater than or lower than the predetermined value, any door of the front and back door shall not be opened.
During the operation of the program, the door safety linkage device will be started, which passes through the pressure sensing mechanism and the micro-switch to make the action of the front door and the back door of the sterilizer fully meet the above requirements.
6)Front and rear door interlock sealing mechanism
The Ministry of Health CSSD recently requires that the sterilization area and the sterile area should be isolated, meaning that the front door is allowed to be opened only if the back door is fully sealed, and vice versa. The sealing of the front and rear doors of this series of equipment is performed by its own solenoid valve and a set of control lines, which fully meet the requirements of CSSD.
7)Compressed gas pipeline pressure control device
The device will off the operation of the program when the compressed gas source pressure is below 0.36MPa. At this time, the screen will be shown in Figure 7, with an alarm sound alarm.

Figure 7
8) Matters need attention
● When closing the door, don't push too hard to break the door switch.
●It is not easy to be too tight or too loose when adjusting the door to the positioning and fixed component, and the tightness should not be automatically open when the door is closed in place.
● When the equipment failure or power failure needs to open the door, we must first see whether the inner chamber pressure is zero, confirm to rotate the manual rod with the ratchet wrench along with the equipment accessories, raise the door, and then open the door.
●When the display area of the touch screen value is displayed as "#", the PLC does not communicate with the touch screen. Please check the communication line.
5.2 Program run and its parameter settings
Click the program selection button on the home page to enter the running program selection interface,The control system has 4 sterilization procedures, 3 detection procedures and 2 auxiliary procedures, as shown in Figure 7. Users can select the application as needed.The following working temperature of each program, sterilization time and so on to do a brief description.| Program type | The sterilization temperature/℃ | The sterilization time/min | drying time/min | holding temperature / ℃ | soaking time/min | The number of pulsations | Pulse upper limit /kPa | Pulse lower limit /kPa | total time /min | ||
| Device program | 134 | 7 | 20 | ---- | ---- | 3 | 60 | -80 | 55 | ||
| The dressing procedure | 134 | 7 | 20 | ---- | ---- | 3 | 60 | -80 | 60 | ||
| Rubber program | 121 | 20 | 20 | ---- | ---- | 3 | 60 | -80 | 60 | ||
| Solid custom | 134 (105-134) | (1-9999S) | (1-9999S) | ---- | ---- | 3 (1-6) | 60 | -80 | |||
| Dry program | ---- | ---- | 15 | ---- | ---- | ---- | ---- | ---- | |||
| BD test | 134 | 3.5 | 2 | ---- | ---- | 3 | 60 | -80 | 40 | ||
| Vacuum test | evacuate: 300s test: 600s | 40 | |||||||||
| PCD test | 134 | 3.5 | 8 | ---- | ---- | 3 | 60 | -80 | 45 | ||
| Enhancement program | 134 | 20 | 10 | 3 | 60 | -80 | 90 | ||||
Table 1
*Liquid program needs to be selected
All application parameters of the device are set in the parameter screen. Press "parameter setting" on the touch screen to pop the user login dialog box. After entering the correct password, press the OK key and the user will log in.
Some parameters already default and you can modify some parameters for each program. First determine the value of each parameter, and then manually touch the input box after each parameter, the set parameters will be displayed in the corresponding display box.
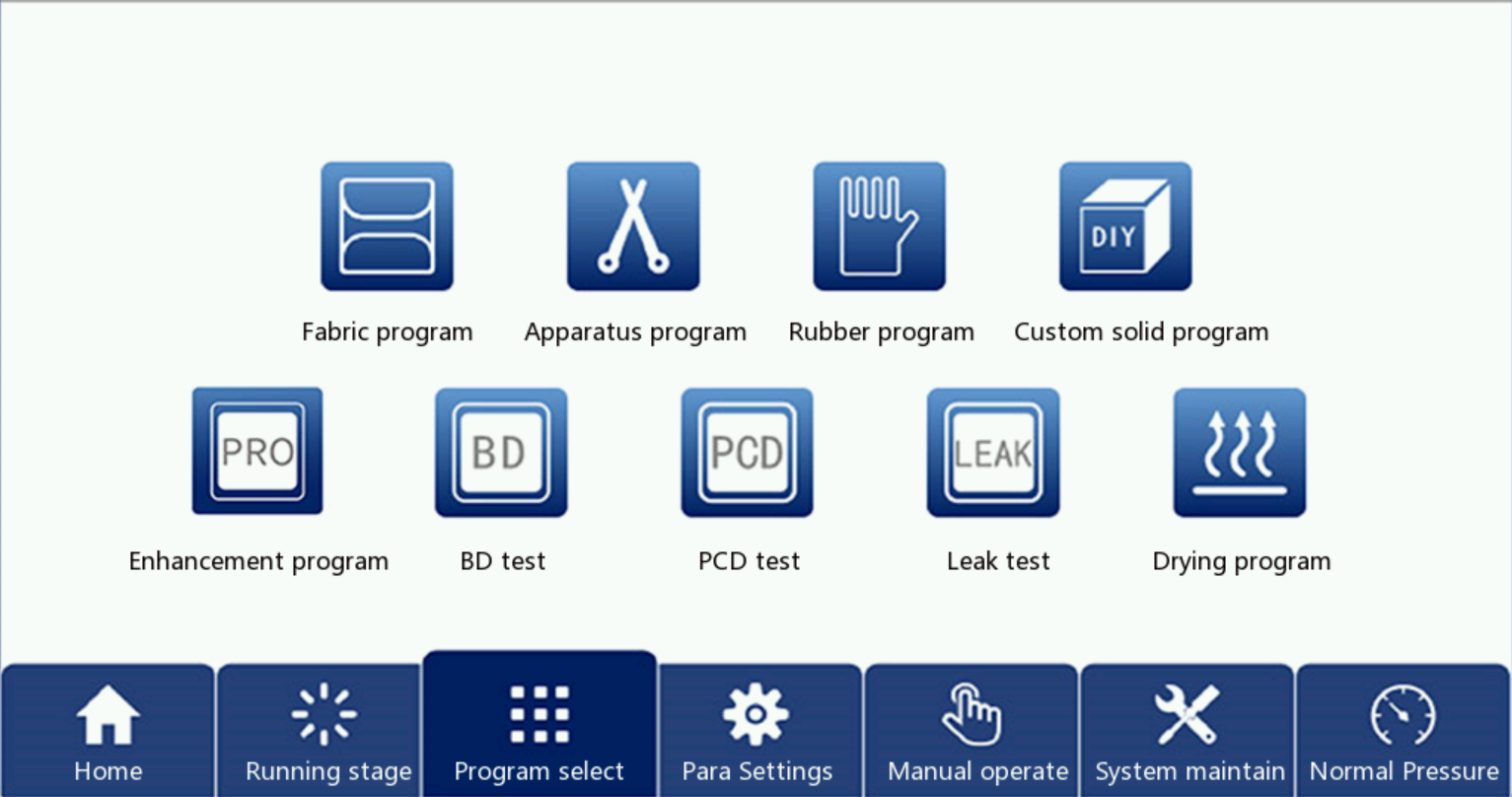
Figure 8
*Liquid program needs to be selected
Here is a brief description of the sterilization items for each program:
| Program type | meanings |
| Device program | Metals such as scalpel, surgical forceps, tweezers and ceramic instruments were sterilized |
| The dressing procedure | Surgical clothes, dressing packs, cotton cloth, masks and other items were sterilized |
| solid body DIY | For solid state articles for sterilization at high temperature, it is necessary to modify the sterilization temperature, sterilization time, drying time, custom sterilization, such as conventional equipment, glassware, rubber, etc |
| Rubber program | Mainly suitable for rubber products, heat-resistant plastics, such as petri dishes and other items sterilization |
| Dry program | Dry the load |
| BD test | Check the penetration effect of the equipment steam and the cold air elimination effect |
| Vacuum test | Check the equipment for leaks |
| PCD test | Test the effect of the equipment on the lumen and cavity-like load sterilizer |
| Enhancement program | Load containing bacteria that are more difficult to kill。 |
Table 2
*Liquid program needs to be selected
The following will be a brief introduction of the program operation procedures and the parameters setting situation commonly used by the device:
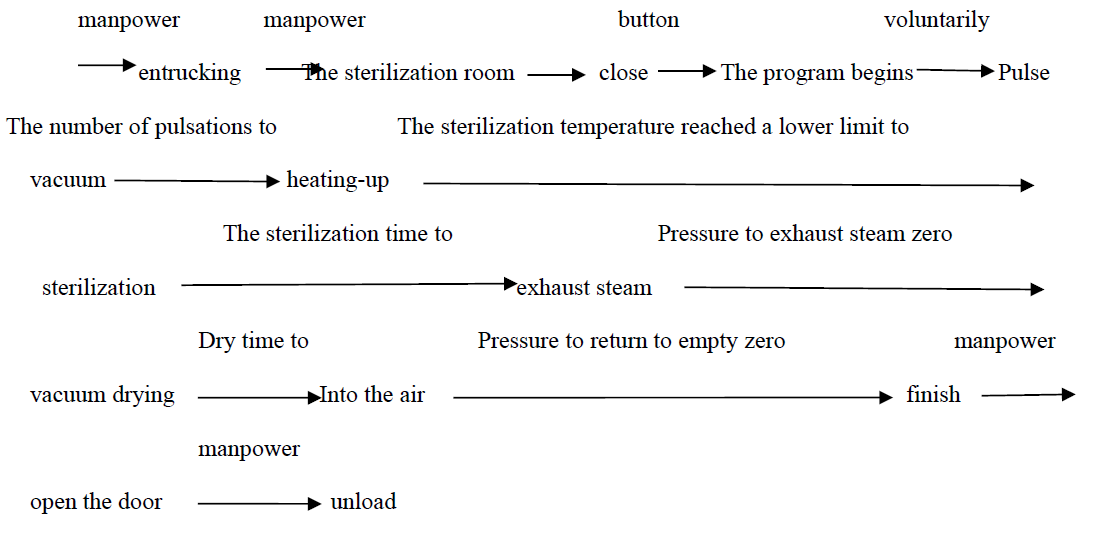
1.Fabric program running procedures and the setting of the parameters
The parameters of the fabric program have been preset in the PLC, and users cannot modify them themselves.
To achieve a satisfactory sterilization effect, each parameter is usually set at:
The number of pulsations:Three times
The sterilization temperature:134℃
The sterilization time:7 Minutes
Dry time: 20 minutes
The number of pulsations——The effect of pulsation is to force out the cold air in the sterilization room and sterilization items through the vacuum pump before sterilization. The amount of cold air exclusion determines the degree of uniformity of the temperature in the sterilization room, so as to determine the sterilization effect. The number of pulsation times and the size of the pulsation amplitude determine the complete air exclusion. According to the calculation, the pulsation can discharge the cold air in the sterilization room by more than 99.2% for three times.
The following table describes the operating state of each control valve and pump during this procedure. The work flow chart of the program is shown below:
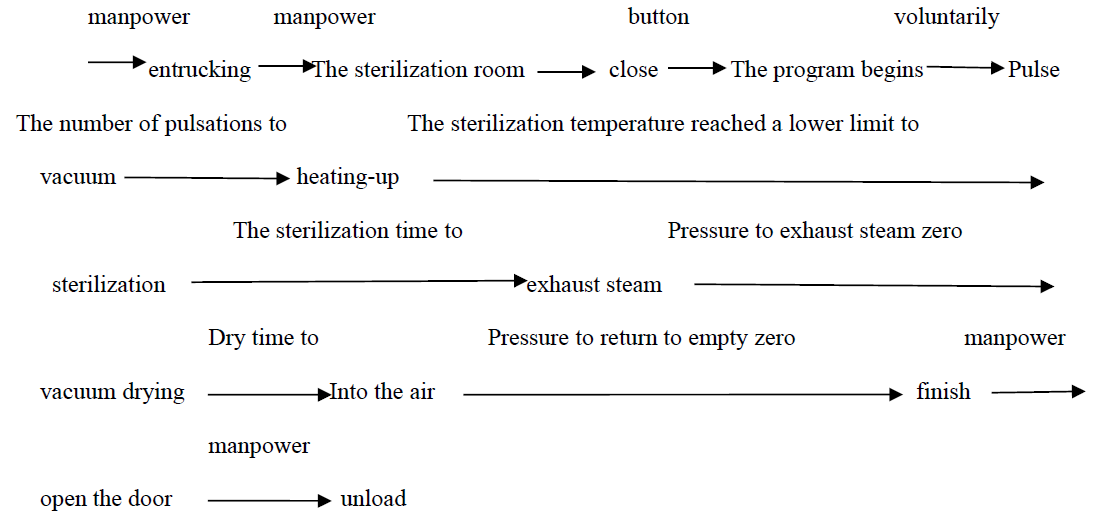
2.Device program running process and parameters setting
The parameters of the device program have been set in PLC and cannot be modified by users. The parameter is generally set to:
Number of pulse movements: 3 times
Sterilization time: 6 minutes
Sterilization temperature: 134℃
Dry time: 10 minutes
The following table describes the corresponding operating state of each control valve piece and pump during this procedure. The work flow chart of the program is shown below:
3.The B-D program running process and the setting of the parameters
The parameters of the BD program are preset in the PLC and cannot be modified by users.
This program parameter value is preset in the program according to the supply room management specification requirements. However, it is slightly different from the equipment or test strip used for BD test; the specific parameters should be modified according to the requirements of BD test strip or test strip manufacturer (the sterilization temperature of BD test ranges from 121 to 137℃).The general preset value (00135 for 3M) is:
Number of pulse movements: 3 times
Sterization time: 3.5 minutes
Sterilization temperature: 134℃
Dry time: 10 minutes
The following table describes the corresponding operating status of each control valve piece and pump during this procedure. The workflow flow chart is shown below:
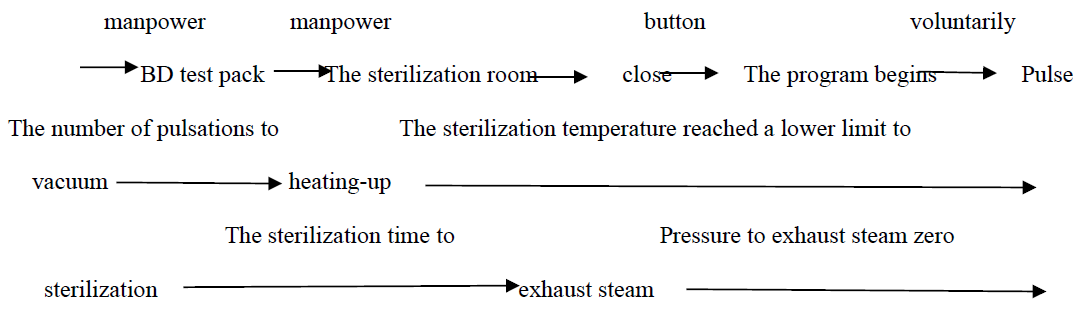
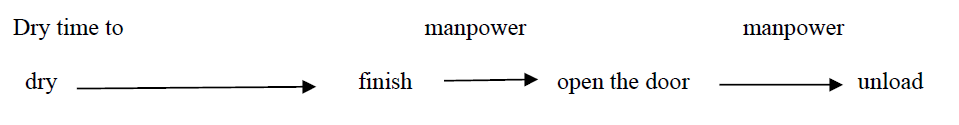
4. Customize the parameter settings
The parameters of the custom program can be modified by users in the parameter screen.
The above is a brief introduction of the operation process and parameter setting of the main programs of the equipment. The other procedures and the above programs are the same, which will not be introduced here. The following is a brief introduction to the program running interface and the start and exit processing of the program.
Click the program selection button on the program selection interface to enter the program startup screen. Take the dressing program as an example to make a brief introduction, click the dressing program button on the program selection interface to switch to the program startup interface. Figure 9
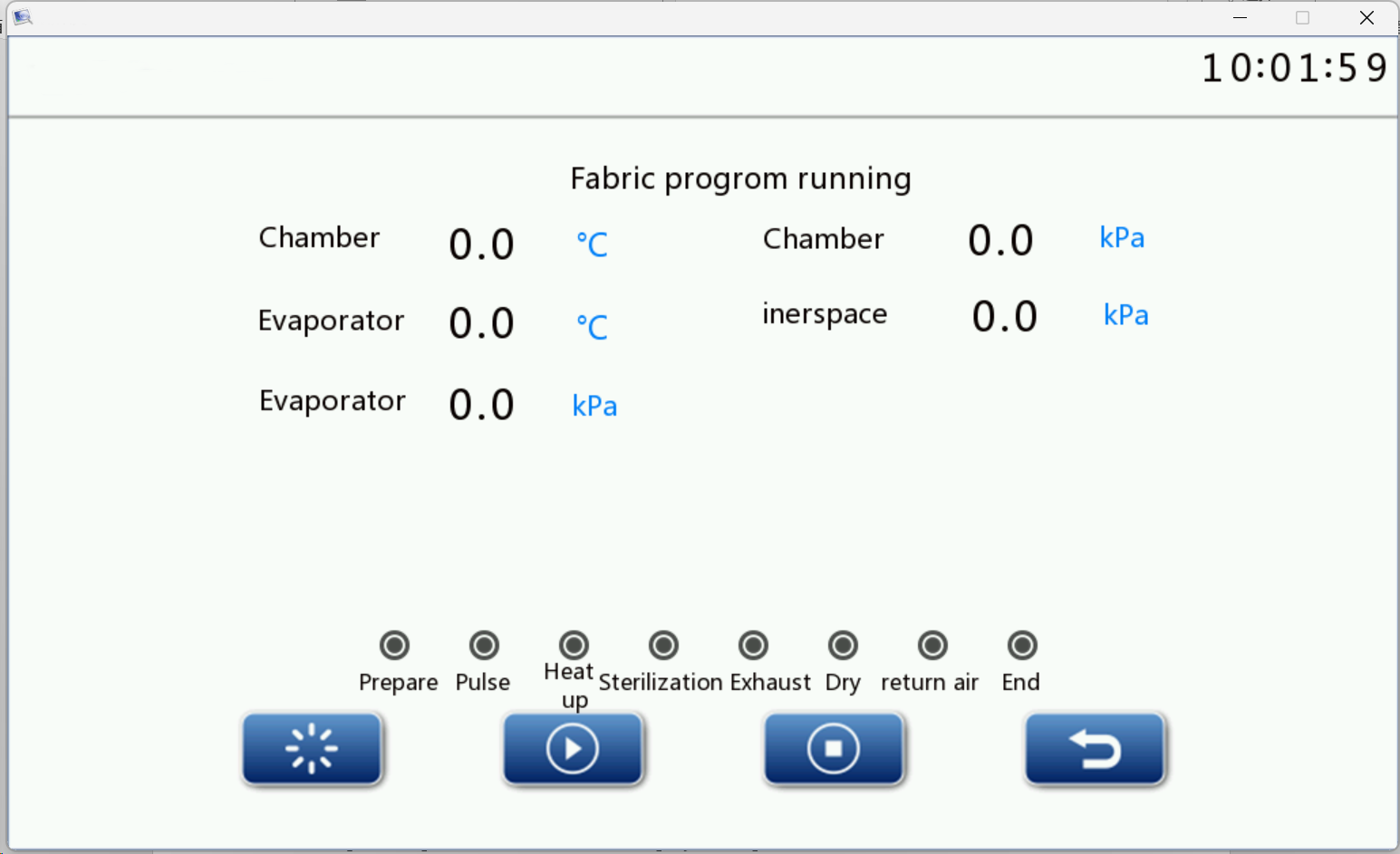
Figure 9
The startup and operation interface can display the operation data such as the inner chamber pressure and the inner chamber temperature in real time. Click the start program button device at the interface to automatically start the program operation, At each stage of the program, clicking the return button interface will return to the previous interface, The automatic program will continue and clicking the Exit program button screen will pop the confirmation interface Click OK to exit the program, and do the subsequent processing after the program exits. After the inner chamber resumes the normal pressure, the display screen and the buzzer will prompt the exit to open the front door, and press the return button to return to the program running interface. Click the running state button will enter the running state interface , the running state interface will display the real-time status of each electromagnetic and pump, and can display real-time value of components such as chamber pressure, temperature, and during the operation of the program, and the pulse times, sterilization time, drying time of real-time display. Clicking the Back button will return to the program running interface.
5.3 Manual manipulation
In order to cope with special circumstances (such as sterilization pressure at the door) and facilitate maintenance and debugging, the system is set for manual operation. The method of entering the manual operation screen is to touch the "Manual Operation" button in the main screen and enter the picture shown in FIG. 10: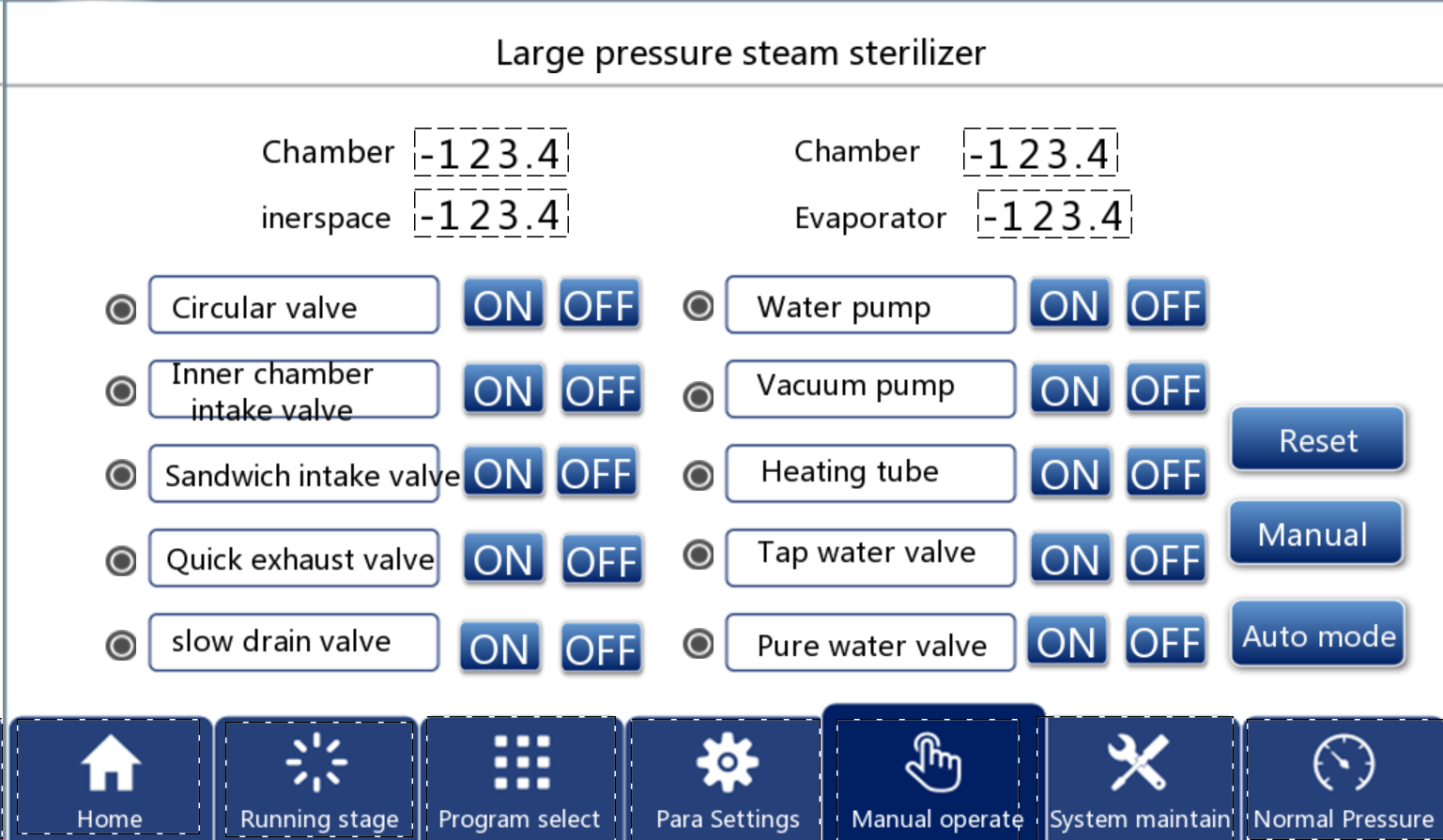
Figure 10
When the device is running automatically running a selected program, the manual function fails, all valves or vacuum pumps cannot be opened or closed by clicking manually clicked, and can only be switched manually when the program ends and the touch screen status indicates a manual status.
5.4 System maintenance
Click the system maintenance button on the screen and enter the correct password to enter the system maintenance interface as shown in Figure 10, this interface has the display of equipment number and equipment operation times, the setting of equipment date, atmospheric pressure, calibration of each temperature, and also contains the entry button of the interface such as alarm record, door maintenance, printing settings.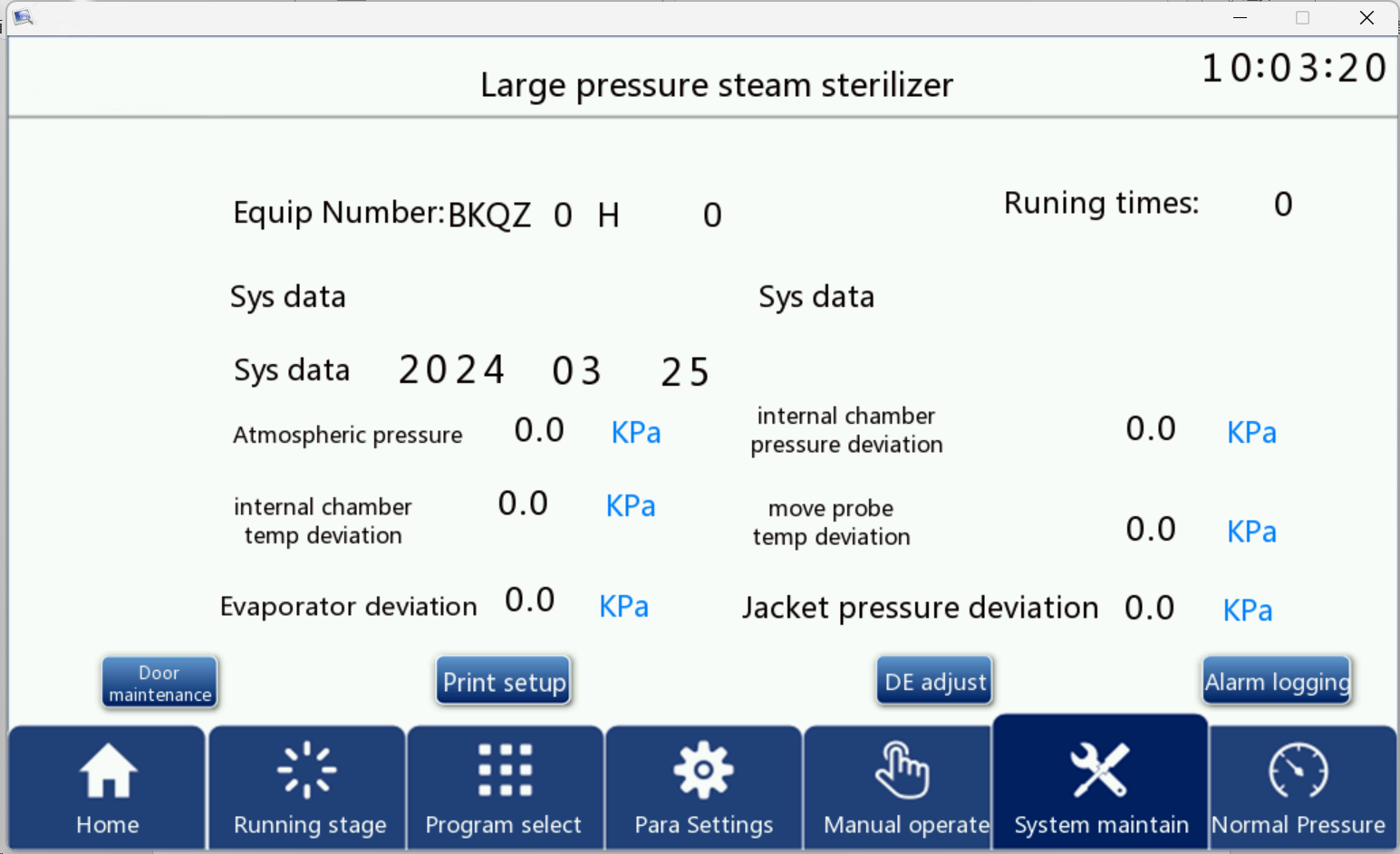
Figure 11
Click the alarm record button to enter the alarm record interface as shown in Figure 12, which can view the alarm record of the device
Figure 12
Click the door maintenance button to enter the door maintenance interface .This interface can give customers the permission to open the back door. When not sterilized, you can click the ON on this interface to open the back door to facilitate the troubleshooting and maintenance of the back door.
Click the print setting to enter the printing setting screen , which allows the user to set the opening of the printing function and the time interval for data printing.
5.5 Parameter setting
Click the parameter settings button on the screen to enter the correct password to enter the parameter settings interface as shown in Figure below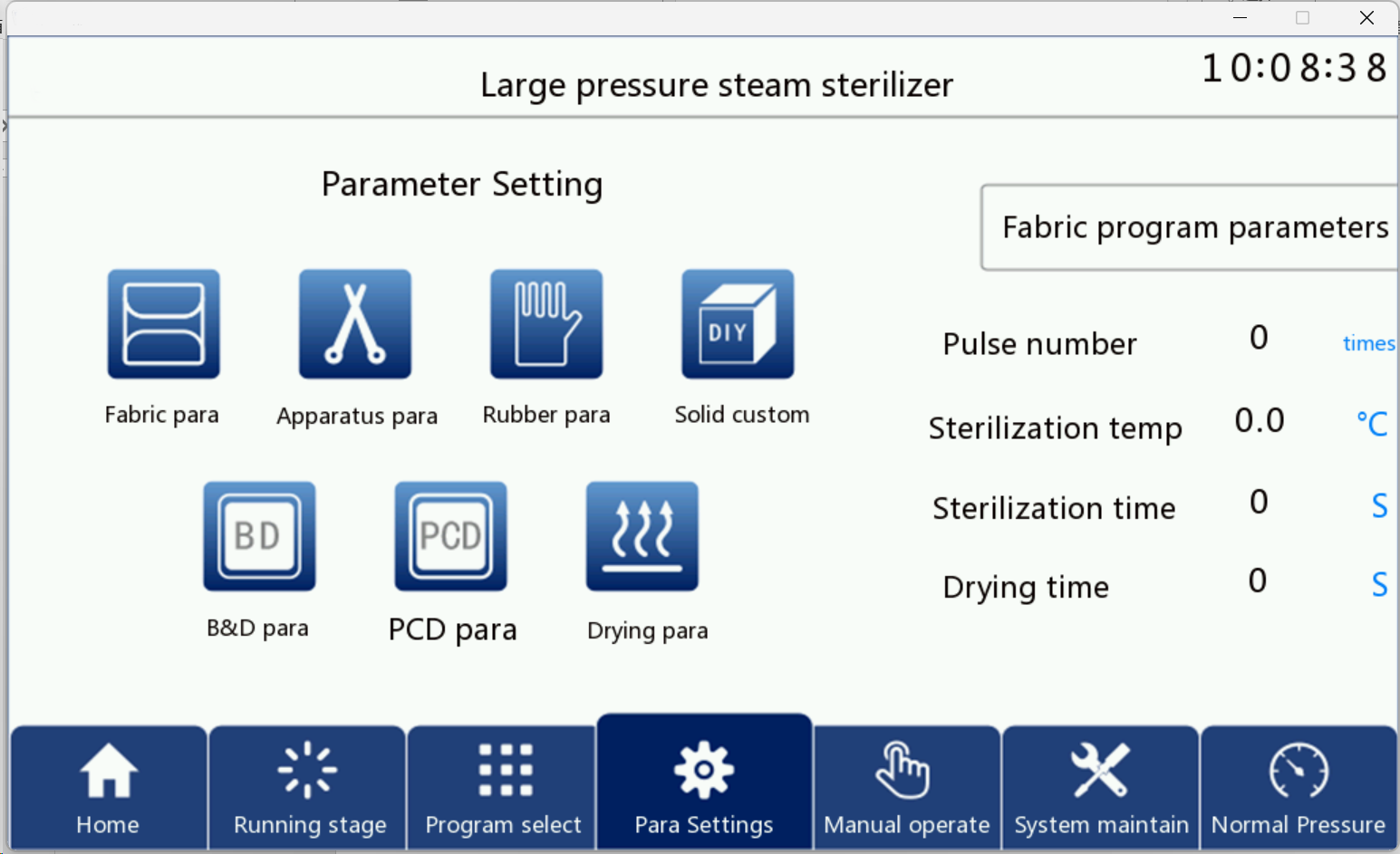
*Liquid program needs to be selected
Figure
Entering the parameter setting interface, users can change and view the program parameters. In order to achieve good sterilization effect, the following program parameters cannot be changed: fabric program, device program, rubber program, BD program, PCD program. And the solid custom and drying parameters customers can change the parameters according to their own needs. Click the corresponding program parameters on the interface to enter the corresponding parameter interface.
Matters need attention:
● During manual operation, when the inner chamber of the inlet valve resumes the normal pressure, the inlet valve should be closed in time to avoid affecting the subsequent operation.
● Do not open the inlet air valve F4 when the inner chamber is under pressure.
● When entering the manual operation, the printer will not print records on the running process parameters.
In the Manual Picture, press the return button and the screen goes to the Main Picture.
6. Daily maintenance, repair and operation procedures of the equipment
The equipment is running normally without any fault, but it should be repaired after a certain time. The fault is not only related to the service life and internal quality of the equipment parts, but also related to the correctness of the use of the equipment and the daily maintenance. Many faults are caused by improper use, incorrect operation, and no attention to routine maintenance and regular maintenance.The correct use and daily maintenance of sterilization equipment are necessary to extend the service life of the equipment and reduce faults. This chapter will provide a brief introduction to precautions and maintenance.
1、Equipment daily maintenance and maintenance
1)Equipment daily maintenance and maintenance
● Turn off the touchscreen power supply.
● Turn off the control power supplies and power supplies.
● Close the steam source valve. Close the steam valve into the sandwich, and the steam in the interlayer will naturally condense and drain from the sandwich trap.
● Close the compressed air valve or power off the air compressor.
● Close the water supply valve.
● Clean the inside of the sterilization chamber (inner chamber) and the sterilization vehicle.
When the temperature of the sterilization chamber and the sterilization vehicle drops to near room temperature, scrub it with a neutral detergent, then rinse it with tap water, and finally dry it with a barren cloth.
Clean up the fiber chips and sediment attached to the front and lower filter of the inner chamber to ensure that the vacuum rate, the smooth flow of condensate water and the temperature indication coincide with the pressure.
 Warning: The cleaning personnel should first enter the inner room, first close the emergency stop switch (power switch), and take the key of the switch with him before entering the inner room to work.
Warning: The cleaning personnel should first enter the inner room, first close the emergency stop switch (power switch), and take the key of the switch with him before entering the inner room to work.● When the sterilizer is not working, open the front door to prevent the sealing performance and service life of the door sealing ring due to long-term compression deformation.
● Check the door seal ring for damage and clean it with a clean soft cloth.
2)Routine maintenance of the main components
● Pneumatic corner seat valve: the pneumatic valve is a powerful on-off valve, all are imported high-quality valve, high reliability, when the use should pay attention to the impact of foreign bodies in the pipeline on the valve parts.
● Liquid ring vacuum pump: vacuum pump uses water ring seal for vacuum. Water rings seal and energy conversion. Because the exhaust has a lot of water in the discharge of steam, so the process of work should be constantly replenished water, but the water should not be too large, too large will increase the power loss of the pump, the working environment of the pump, the water according to the actual situation by the pump through the automatic absorption of the water tank. Water quantity and water temperature directly affect the vacuum rate and the life of the pump. The lower the water temperature, the higher the ultimate vacuum degree, and the general maximum requirement does not exceed 25℃. When stopping for a long time, you should open the pump bottom screw plug, put the pump memory water clean, and then block the water release hole, filled with saponification liquid. Otherwise, the pump is easy to rust or freeze crack pump body, affecting the pump efficiency and service life.
● Hydrobic valve (pneumatic corner seat valve, solenoid valve): the internal chamber exhaust pipe has a small corner seat valve for the internal chamber drainage, the sandwich exhaust valve has a small solenoid for jacket drainage, the drainage work is normal or not directly affects the sterilization effect. If the water cannot be discharged normally, the drain valve should be opened for cleaning. At work, a small amount of steam discharge is a normal phenomenon.
●Safety valve: Install one safety valve each in the jacket and the inner chamber. On the sterilization device to protect the equipment and personal safety, jacket safety valve installed above the cabinet, the inner chamber safety valve on the side of the cabinet, the opening pressure and back pressure has been adjusted when the factory, the user do not adjust to avoid accidents due to improper adjustment, but need to half a year to its hand pull several times, wash with steam, to prevent its parts for a long time not action corrosion, when sterilization overpressure safety valve working for some reason.
 Warning Remember not to contact the safety valve handle directly by hand, use a screwdriver or tie a cloth to pull, to prevent steam damage to the operator!
Warning Remember not to contact the safety valve handle directly by hand, use a screwdriver or tie a cloth to pull, to prevent steam damage to the operator!● Pressure regulating valve (if optional): when leaving the factory, the inlet steam pressure regulating valve has been adjusted, the user if there are special requirements, can readjust. The two film boxes of the pressure regulator are prone to stretching or compression for a long time, easy to welding and corrosion, if found, steam leakage should be replaced in time.
● Debacterial air filter: the filter works in the vacuum breaking stage, and has the characteristics of high filtration accuracy, energy saving, safety and reliability. The gas filtration accuracy can effectively solve the repollution of the sterilized items after entering the air into the inner chamber, and the filter element can be used for one to two years in general.
● Filter: each steam inlet and the inlet pipe has a filter, its function is to filter out the impurities in the steam pipe and the inlet pipe, to ensure the subsequent pipeline and the reliable action of each valve, so it is necessary to be cleaned regularly to prevent blockage. When cleaning, screw out the lower plug, clean the filter screen, and then tighten the plug. In addition, one filter should be installed in front of the trap and the inner chamber trap. These two filters are crucial for the normal trap of the jacket and the inner chamber, so the filter should be removed and cleaned regularly.
● One-way valve (check valve): the door evacuation pipe is equipped with a one-way valve, which should be checked regularly, to avoid foreign bodies affecting its one-way sealing performance. The simplest way is to use the nozzle suction test after cleaning, no feeling of air leakage is normal.
● Solenoid valve: in order to make the solenoid valve work normally, the solenoid valve valve core and valve seat must be scrubbed once a season, to prevent the impurities in the pipeline from affecting its opening and closing.
Note: Check whether the solenoid valve is electric, you can use a screwdriver to test the nut on the valve head for magnetism.
● Condenser: pay attention to the quality of water, excellent water quality will condense a large amount of scale inside the condenser, will affect its condensation effect, so it should be chemical scaled according to the situation. For a long time shutdown, unscrew the lower part of the condenser, clean the water stored inside, and then plug the water hole.
3)Maintenance of the electrical components
● Electrical components and connections are strictly prohibited to contact with the water. If the water is accidentally stained with the water, the power supply should be turned on only after the treatment.
● It must be dust-proof and dust once a season.
● The plug seat of each connection, each plug element should be often checked for loose, loose should be tightened.
4)Removal of scale
Because the water quality in some areas is too hard, the long-term operation of the equipment will deposit a large amount of scale in the pump and in the pipeline, and the accumulation of scale will affect the normal operation of the equipment. It is recommended to use water sources that meet the boiler water standards or deionized water, and use all kinds of chemical descalers for comprehensive descaling treatment regularly (recommended once half a year).
Scaling process:
① Remove the water from the vacuum pump and the condenser first.
② There is a flange connection at the drainage outlet of the vacuum pump, remove the flange screw, pour the chemical scale removal agent into the vacuum pump from the vacuum pump, fill the vacuum pump, the condenser and the pipe connected with the inner chamber, and wait for about 4 hours (if other types of cleaning agent is used, it should be handled according to the requirements in the instructions of the cleaning agent).
③ Take the JT-100 water system safety descale cleaning agent as an example: mix the cleaning agent and water in the ratio of 1:10 (if using other types of cleaning agent, then follow the proportion required in the cleaning agent instructions), and then open the vacuum pump to discharge the water into the sewer. Finally, pour water into the pump to rinse the residual cleaning agent.
Note: During descaling operation, the cleaning agent must contact the human body to burn skin!
2、Daily operation procedures of the equipment
1) Prepare the equipment before use:
① First, clean the condensate water in the steam pipe, and then open the steam source and water source switch connected with the sterilizer, and check whether the steam source pressure reaches 0.3-0.5MPa, and whether the water source pressure reaches the specified 0.15-0.30MPa value.
② Open the air compressor power supply and open the compressed air valve when the pressure reaches the specified value.
③ Close the power supply and control power supply of the equipment, dial the sterilizer power switch to the "-" side, and preheat the equipment to prepare the equipment for the program operation.
④ Mark the operator's name and date on the B-D test paper, and put it into the sterilizer to run the B-D test program, to test the equipment without leakage, and whether the equipment is normal.
⑤ Organize the sterilization package, binding is not easy to be too tight, external chemical indicator tape, built-in chemical indicator card.
⑥ Check sealing ring, front seal and door panel for debris and damage and scrub with clean cotton cloth.
2)Vibrator operation:
① After passing the B-D test, open the sealed door, and push the sterilization vehicle already loaded with the sterilization items into the sterilization room.(There shall be a gap between the bag and it is not attached to the device wall and door panel)
② Close the sealed door, select the sterilization program according to the sterilized item, check whether the sterilization parameters are correct, and start the operation procedure.
③ In the process of sterilization, the operator should not stay away from the equipment, should closely observe the operation of the equipment, if any abnormal, handled in time, to prevent accidents.
④ Do a good job of sterilization effect detection, record and archive, to facilitate tracking and investigation.
⑤ After the sterilization, after the indoor pressure back to zero, can open the back door to take out the items.
⑥ After the sterilized items are removed from the sterilizer, they should be placed on the shelf to prevent secondary contamination.
3)The equipment works after use:
① Open the front door, press the power emergency stop switch, and cut off the equipment control power, power power and air compressor power.
② Close the steam source, water supply valve and compressed air valve.
③ After the daily work, the inside and outside the sterilization and the operating room should be kept clean, the sterilization room should be cleaned,
Once a week small maintenance, once a month big maintenance. The drain valve should be cleaned once every three months, and the filter on the water inlet steam and the water inlet pipe should be cleaned once every half a year to prevent impurities from blocking.
7. Common Faults and Solutions
7.1 Common fault information
| Phenomenon | Possible cause | Correction method |
| The power switch is turned on, and the power indicator light is not on | 1. Circuit breaker is not closed 2. The main power supply switch is damaged | 1. Close the circuit breaker 2. Replace the power switch according to the specific circumstances |
| Heating up state, the pressure, temperature does not rise or rise slowly | 1. The control circuit of the heater is short-circuit or burned down 2. Serious leakage at the pipe joint or safety valve | 1. Check and replace the damaged devices 2. Check and tighten the pipe joints, safety valves, etc |
| Drainage state, the pressure, temperature does not drop or drop slowly | The drainage pipe is blocked or bent or blocked | Check the drainage pipe |
| No sterilization temperature was reached | Is it the boiling point of the altitude at? Check confirm the setting temperature of the boiling point | Please contact us or your agent |
| The safety valve is opened | 1. Is the pressure too high? 2. Is the safety valve faulty? | 1. Adjust the temperature deviation 2. Correct and replace the safety valve |
| The door leak | Is the door glue ring getting hard and aging? 2. Is the door tape cracked? 3. Does the door rubber ring fall off? | 1. The door rubber ring must be replaced 2. The door rubber ring must be replaced 3. Reinstall the door glue ring |
Table 3
7.2 Alarm code
When an error occurs, the error code displays and the buzzer calls to alarm, and the sterilization automatically stops the operation. Please find the following situation and handle it. If there is a fault, please wait for the equipment to step down before contacting the equipment. In case of a special uncontrollable or unknown situation, any person can press the red emergency stop button on the control panel of the right control side of the sterilizer to make an emergency close, then cut off the external switch of the equipment, and contact the technical personnel of our company for handling guidance.Alarm information control table
| No. | Alarm code and its contents | Cause of production | Solution |
| 1 | E02 Exit halfway | Select Stop and exit the program during the program running | Wait for the device to exit |
| 2 | E03 The front door is not closed! | The front door is not closed or the detection switch is not detected while running the procedure | Check the front door lock closure and switch wiring |
| 3 | E04 The rear door is not closed! | The rear door is not closed or the detection switch is not detected when the program is running | Check that the rear door lock is closed and close the switch wiring |
| 4 | E05 Front-door motor overload | Equipment front door motor overload | Press the corresponding door motor thermal relay reset button to restart the equipment and check for obstacles during the motor operation. |
| 5 | E06 Rear door motor overload | Equipment rear door motor overload | Press the corresponding door motor thermal relay reset button to restart the equipment and check for obstacles during the motor operation. |
| 6 | E07 Low compressed air pressure! Please replenish gas in time. | Low compressed air pressure | Check whether the air supply pipes and equipment are normal. |
| 7 | E08 Opening the front door timeout! | The front door opens for too long | Check the front door motor and front door in switch. |
| 8 | E09 Opening the rear door timeout! | The back door opens for too long | Check that the rear door motor and rear door switch are normal. |
| 9 | E10 The pressure in the equipment is too high, exit halfway! | The pressure inside the device exceeds the preset pressure | Check that the exhaust solenoid valve is normal Check the equipment pressure detection parts or contact our technicians |
| 10 | E11 heat-up timeout | The heating phase time exceeds the preset time | 1. Check the equipment for obvious steam leakage 2. Check whether the exhaust solenoid valve is not tightly sealed And continue with the cleaning 3. Check whether the evaporator is normal |
| 11 | E12 The sterilization temperature in the inner chamber is low, so exit midway! | The inner chamber temperature was lower than the sterilization temperature during the sterilization process | 1. Check the exhaust solenoid valve 2. Check the temperature detection parts of the equipment or contact our company technicians |
| 12 | E13 The temperature of the inner chamber is too high. Exit halfway! | The temperature of the internal chamber was above the sterilization temperature of + 3 C | 1. Check the air intake solenoid valve 2. Check the temperature detection parts of the equipment or contact our company technicians |
| 13 | E14 Vacuum pumping timeout, exit halfway! | The vacuum does not reach the predetermined lower limit within the specified time | 1. Check the exhaust solenoid valve 2. Check whether the door glue ring is dirty and clean it |
| 14 | E15 Cavity pressure failure | The inner chamber pressure is out of normal range | Check pressure sensor |
| 12 | E16 Dry burn the heating pipe!!! | Output signal of the heating pipe protection component | Check the heating protection components, heating control and water level |
| 13 | E17 Water supplement timeout, exit halfway! | Low water level was detected during the operation | 1.Check whether there is water in the external pure water storage container, or whether the external pure water machine water supply is sufficient 2. Check whether the water inlet valve and pump are working normally |
| 14 | E18 Cavity temperature failure | The luminal temperature was detected as not in the normal range | Check the temperature detection component or contact me Company technical personnel |
| 15 | E19 Moving temperature fault | The moving temperature sensor was detected as not in the normal range | Check the temperature detection component or contact me Company technical personnel |
| 16 | E20:Phase sequence error, please change the phase sequence!!! | The device input power phase sequence error is detected. | Please change any two connections. |
| 17 | E22:The vacuum pump is overloaded | The vacuum pump is overloaded. Procedure | 1. Check whether the tap water is insufficient. 2. Check whether the vacuum pump is blocked. 3. Contact our technical staff |
Table 4
7.3 Analysis and exclusion of wet packets
 The weight of the dressing pack is 3% higher after sterilization than that before sterilization, which is called the wet pack phenomenon.
The weight of the dressing pack is 3% higher after sterilization than that before sterilization, which is called the wet pack phenomenon.• Loading problem: whether the loading is too large so that some packages contact the sterilization indoor wall, and the condensate water seeps into the package. Check whether there is any water vessel in the bag, so that the condensate water can not be discharged in the sterilization process.
• Poor drainage pipeline: check whether the drainage pipe has too many corners, from low to high discharge, whether there are impurities in the pipeline blockage.
• The check valve of the inner chamber drainage pipeline is damaged: this is manifested as wrapping the outer wet and inner dry, and the water from the drainage pipe returns to the inner chamber during the drying process.
• Drying time is too short: increase the drying time appropriately to observe whether the drying effect is improved.
• The negative pressure does not meet the standard: refer to the "sterilization device fault and troubleshooting table" for inspection.
• The local wet bag should be analyzed according to the specific location of the wet bag and investigated.
7.4 Analysis and exclusion of unqualified sterilization
 The three major elements of sterilization are: saturated steam, sterilization temperature and time.
The three major elements of sterilization are: saturated steam, sterilization temperature and time.• Cleaning of items: check whether each cleaning process is strictly operated in accordance with the workflow of the supply room to ensure the quality of cleaning.
• Packaging of goods: the packaging should not be too large, too tight, whether the packaging material is breathable and other factors will affect the elimination of cold air and steam penetration.
• Loading principle: whether the loading is correctly placed according to the provisions, whether too tight, too large amount and affect the smooth flow of steam.
• Residual of cold air: the amount of cold air determines the effect of disinfection and sterilization, so we should focus on checking whether the performance of sterilization equipment is normal. First use B-D test paper for detection, if there is a problem, conduct pressure holding experiment or use manual operation to connect the inner room into the steam inspection pipeline and there is no leakage.
• Sterilization temperature: use the retention point thermometer to check whether the actual temperature in the package reaches the sterilization temperature, whether it is consistent with the display temperature, check whether the inner chamber pressure and the temperature correspond to each other, and whether the observation results of the appropriate increase in the inner chamber pressure are improved.
• Sterilization time: whether the sterilization time is reasonably set up, whether the inner chamber inlet steam is too fast, the penetration time is not enough, and appropriately extend the time to observe whether the results are improved.
• Fault of sterilization equipment: the system refers to sterilization failure and troubleshooting table.
• Improper detection method, repeated detection or the use of other batch number of reagents.
8. Quality assurance measures for sterile products
Any kind of disinfection and sterilization equipment, whether the exhaust or pulsating vacuum equipment, to obtain reliable sterilization effect, in addition to the equipment itself design, manufacturing quality and the perfect maintenance of the equipment, for sterilization staff correctly grasp the basic knowledge of disinfection and sterilization, familiar with the working principle of equipment, strict disinfection sterilization work operation specification, seriously treat each regulation related to sterilization, is an important human factors for sterilization success. Warning: Sterilizing equipment works at high temperature, special attention should be paid to prevent scald.
Warning: Sterilizing equipment works at high temperature, special attention should be paid to prevent scald.1、The sterilized articles must be cleaned before sterilization
The cleaning treatment of sterilized items before sterilization is the key to successful sterilization, especially some difficult medical supplies. Foreign studies have found that thorough cleaning can reduce the bacterial amount by 3-4 logarithms to reduce the content of organic matter; If the organic matter cannot be effectively removed, it will greatly reduce the activity of the sterilization agent, and the bacteria hiding in the organic matter are not easy to be killed by the sterilization agent; so if the cleaning is not thorough, the whole sterilization process may fail. It must be noted that the cleaning requirements cannot be reduced by extending the sterilization time and increasing the temperature of the sterilization agent;In order to facilitate the effective removal of organic matter, it is best to use enzyme-containing cleaners to enhance the effect of cleaning. Cleaning is divided into manual cleaning and machine cleaning. Manual cleaning is time-consuming and laborious, and it cannot guarantee the consistency of each cleaning. In addition, the medical personnel during the manual cleaning must wear waterproof masks, eye masks, gloves, sleeves, hats, waterproof shoes, aprons, etc.;Machine cleaning is a large investment, but can ensure the consistency of cleaning; but it must be noted that: machine cleaning can not completely replace manual cleaning, for some pipes, fine instruments and more difficult cleaning parts must be manually cleaning; in addition, the cleaning equipment must be often cleaned and maintained to avoid machine cleaning failure.
 Warning Without proper cleaning, a high level of disinfection and sterilization effect cannot be guaranteed.
Warning Without proper cleaning, a high level of disinfection and sterilization effect cannot be guaranteed.2、Quality of steam source
● Saturated steam: saturated steam is steam where vaporized water rises above the liquid phase line.
●Steam dryness: it is another requirement of the nature of steam. According to the practice knowledge, the content of saturated steam should not be less than 97%. That is, water particles or unclean substances should be less than 3%. Otherwise, it is easy to cause the wet packet phenomenon.
3、Water quality control
Water hardness is divided into two categories:First, the temporary hardness is mainly formed by the heavy carbonate of calcium and magnesium, and second, the hardness is formed mainly by the sulfate, nitrate and chloride of calcium and magnesium, which cannot be removed by boiling, so it is also called the permanent hardness. Because the disinfection and sterilization equipment works under hot and humid conditions for a long time, the impurities in the water, such as dissolved oxygen, carbon dioxide, hydrogen sulfide, chloride and so on, whether gaseous or particles, can cause intercrystalline corrosion of stainless steel and metal. Therefore, the PH value of the water supply and steam supply for disinfection and sterilization equipment is required to be between 7-7.8, and the water used for boiler and sterilization equipment must be softened.
4、The environment of the sterilization room
● The ground of the disinfection and sterilization room must be smooth and smooth, and the cement ground is appropriate. The wastewater discharge outlet is slightly inclined to keep the operation area clean and dry. The roof should be provided by the ceiling to prevent dust from gathering. The walls shall be flat and clean, and isolated from the studios such as washing and preparation.
● The discharge outlet of waste steam and waste water must be discharged through the wall or buried in the trench, or discharged from the atmosphere; no residual water and steam will return into the interior.
● Install ventilation devices properly at the top of the wall or at the ceiling.
● Conditional for equipment partition, because the sterilization equipment using high temperature steam as sterilization medium, use vacuum pump vacuum, air compressor for door seal and pneumatic valve to provide compressed gas, so will cause certain impact on the surrounding environment, the equipment after placement with color steel plate or other materials operating end —— front door and non-operating end —— back door partition (single door sterilizer only front door partition), this will effectively prevent the impact of heat and working noise on the surrounding environment, greatly improve the working conditions of disinfection and sterilization room.
5、The sterilized items are packed, prepared and placed on the sterilization vehicle
● The dressing fabric should be folded and wrapped to facilitate steam penetration.
● When the package is placed in the sterilization vehicle, the adjacent package room shall keep a 10mm clearance, and the loading capacity shall not exceed 80% of the inner chamber volume, so as to facilitate the smooth flow of steam.
● Commercially available aluminum lunch boxes and enamel boxes shall not be used for storing items to be sterilized, but shall be installed with appliances with standard sterilization boxes or ventilation holes.
● A batch of similar items should be sterilized as much as possible, and avoid the direct contact with the cotton bag. If different types of items must be placed together, the temperature and time required for the most difficult item to achieve the sterilization shall prevail.
●The package should not be tied too tightly, use the chemical indicator tape seal, sterilization the bag in the chemical indicator card.
● During placing, plates, basins, bowls and other metal vessels should be placed vertically; fiber substances should be folded in a vertical direction to the horizontal state; glass bottles and glass tubes should be opened down or side to facilitate steam entry and air removal.
● Cloth and metal items are sterilized at the same time, the metal items pack should be placed under the dressing pack, so that the two heat is basically the same, and prevent the condensation water produced in the sterilization process of metal articles from wetting the cloth pack.
● Difficult to sterilize the big bag should be placed in the top layer, easy to sterilize the small bag in the lower layer.
6、Containers and delivery tools shall be cleaned and disinfected daily.
9. Detection of Sterilization Effect
The detection of the sterilization effect is a means to evaluate whether the sterilization method used for the sterilized articles is reasonable and whether the sterilization effect is reliable.Customers should regularly verify the sterilization effect and monitor the sterilization effect, physical mode and chemical testing equipment should be tested every time. Biological testing customers can test according to the needs, and it is recommended to conduct biological testing at least once between every 5-10 cycles. Specific tests are as follows:
Detection methods can be divided into three categories: physical detection, chemical detection and biological detection:
1、Physical way
1) Instrument detection (process monitoring): This series of equipment is equipped with a pressure gauge showing the interlayer and the inner chamber pressure, and displays the inner chamber temperature and pressure value on the touch screen, the equipment is equipped with a micro printer (the microcomputer monitoring system is not equipped with a micro printer), the printer can print out sterilization data record paper for archiving for reference. The recording paper can record the pressure, temperature and corresponding time value of each sterilization procedure in the sterilization process, and can also record the operation times of the equipment, the nature of the use procedure, the number of pulsations, the sterilization time, the drying time, the sterilization temperature and other parameters, and preliminarily judge the sterilization effect by observing whether these values are consistent with the requirements.
2)Thermocouple detection: the method is the thermal electric couple electrode into the sterilizer to test parts, close the cabinet door and lead, by the verification instrument data analysis, by the computer will detect the temperature of each point timely display, using the method can detect the sterilization chamber temperature uniformity and the stability of sterilization temperature, or directly with the radio directly in the sterilizer test. In order to increase the reliability of physical monitoring, it is necessary to strengthen the debugging and verification of testing equipment, and combine chemical testing and biological monitoring to comprehensively analyze the quality of sterilization.
2、Chemical detection method
The characteristics of heat discoloration or deformation of chemical indicators under a certain temperature and time of action are used to determine whether the parameters required for sterilization are reached.
1) Chemical indicator card: this kind of indicator card is used to detect the sterilization effect of the pulsating vacuum sterilization device. The indicator color block on the indicator card will be changed from light yellow to black during the sterilization process. Whether the sterilization results can be determined from the depth of the color change. The class of indicator cards has the chemical indicator cards for the detection of the pressure steam sterilization effect at three temperatures of 115℃, 121℃, and 132℃. When use will indicate the temperature, and can indicate the temperature duration of the chemical indicator card inside the sterilization package, after a sterilization cycle, take out the indicator card, compared with the standard color, if the indicator color deeper than the standard sterilization qualified, conversely, said the temperature and duration did not meet the requirements, need to sterilization. Indicates that the water wet can affect the accuracy of color change, so try to avoid contact with metal, glass and other surfaces easily produce condensate items.
2)Chemical indication tape: The tape is coated with adhesive on one side and a chemical indicator on the other side. It can be used both as an indication to distinguish "sterilized" from "unsterilized" and as a package seal on the appearance of the dressing package. After sterilization, the success of the package can be preliminarily judged by the uniform blackening of the indicator color.
3、Biological detection method:
Biological detection refers to the use of live microorganisms to test the sterilized articles to identify whether all the microorganisms in the sterilized articles die, and to assess whether the sterilization equipment is qualified.
● Calibration indicator bacteria: use the internationally recognized "thermophilic lipobacillus spores", the most difficult to kill and heat resistant, as the sterilization indicator.
● Medium: The test medium is bromocreol violet protein water medium.
●Detection method: place the 3M produced 132℃ (121℃) biological indicator in the middle of the standard test package (package size 220mm 300mm 250mm). The test bag was placed above the exhaust port in the sterilization room. Under the sterile conditions after the sterilization procedure, the standard test bag was removed and placed into the 3M culture dish for 48 hours to observe the color change of the culture medium. Negative and positive controls were tested.
In addition, other testing equipment and articles approved by the Ministry of Health can also be used to operate according to the instructions of the manufacturer.
●Results: The color of all medium was unchanged and qualified. If you change from purple to yellow, that is, sterilization failure. If other testing equipment and articles are used, the sterilization effect can be determined according to the manufacturer's instructions.
● Treatment: If the sterilization is not qualified, the articles can be temporarily sealed, find out the possible cause of sterilization failure, and then re-sterilized; also retest the sterilization effect of the same biological indicators of the same manufacturer; carefully check the production date, effective date, any damage and contamination during culture.
● The equipment shall be put into use before normal use or after passing the biological test for three consecutive times after overhaul.
● The test results shall be recorded, including: inspection date, sterilizer number, sterilization temperature, sterilization time, indicator source, batch number and validity period, culture temperature, culture time, observation result and examiner.
 Warning After sterilization, the tablets should be timely removed according to the manufacturer's instructions for bacterial culture treatment.
Warning After sterilization, the tablets should be timely removed according to the manufacturer's instructions for bacterial culture treatment.Physical detection, chemical detection, biological detection of these three methods have their own different purposes and significance, so they cannot be replaced by each other, and should be used in combination with each other.
Physical test —— can explain the operation status of the sterilization equipment itself, and can directly show whether the time, temperature, pressure and other related sterilization parameters in the working process are normal, so as to preliminarily determine whether the sterilized items have been sterilized successfully.
Chemical detection —— can detect whether the sterilization process is completed, can understand how the penetration of steam to the package, can provide the first visual inspection at the moment after the completion of sterilization, to assist in judging the effect of sterilization.
Biodetection —— is used for the final discrimination of the sterilization effect, but it is costly and long, making it impossible to use it per package or per pot.
4、The Bower-Dick Test (the B-D Test)
The B-D test was designed in 1963 by two Scottish microbiologists, Bob and Dick, specifically to test the effect of air exclusion in a vacuum pressure steam sterilizer.
● B-D test map: A mixture composed of a variety of chemicals is made into an indicator ink through a certain carrier. The indicator ink is printed on a special size of paper with a certain air permeability, forming a certain pattern, and it becomes a B-D test vacuum test map. The chemical indicator on the test map is sensitive to residual air and can detect the presence of residual air in the test package.
●B-D test: This test shall be performed before the first sterilization per day. The test is to place the B-D test map in the middle of the test bag (also use the disposable B-D test bag), then put the test bag at the exhaust outlet of the sterilization room, close the sealed door, set the parameters according to the instructions of the manufacturer of the B-D test bag, run a sterilization procedure, open the door, unlock the test bag, remove the test diagram, and observe the test results.
● Preparation of the test bag: The test bag consists of a pure cotton cloth towel of the size of 46-50cm and 80-90cm, which is first horizontally folded into three layers, and then vertically folded into six layers.stack folded cloth to a height of 25cm. When stacking, each layer of cloth towel is placed alternately on the left and right sides of the folded side, so that the thickness of both sides is equal. After the cloth towel is placed, the B-D test drawing holder is placed between the central cloth towel, and then wrapped in the cloth towel. The outside cloth is fixed with the chemical indicator adhesive tape to become a test bag. The whole test package is about 27-30cm long, about 23-25cm wide, and about 25-28cm high, and the weight is about 4-5Kg.
● Results judgment: the test map becomes black and uniform, that is, the central part and the edge part of the same color, indicating that the air is completely discharged, the sterilizer vacuum system is good, each part sealing condition is good, indicating that it can be used. If the test diagram is uneven, and the central part is usually lighter than the edge part, indicating that the air is completely excluded and the sterilizer performance is poor, it can be used after repair.
● Note: cloth should be washed, but not hot, because excessive drying will affect the test results; reusable test bag, cloth must be washed, continuous test, each should open the bag, cloth dry for 1 hour, then repackaging, cloth too wet can also affect the test results; the package should be loose, not too tight or too small.
● Preferences: name of the sterilization package; sterilization date or failure date; name or code of the instrument examiner and the packer; sterilization mark at the seal.
● The results only indicate the vacuum state of the equipment and the residual amount of cold air, and do not mean whether the sterilized items are qualified.
 Warning
Warning1、 The contents and attached charts in this manual shall not be misappropriated without the permission of our company.
2、 With the development of The Times, the design and process of equipment will be updated, the company reserves the right to modify the contents of this specification.
3、electromagnetic compatibility
The equipment is applicable to medical, health institutions such as hospitals, CDC, as well as factories and laboratories, and meets the launch and immunity requirements of GB / T18268.1. The equipment is designed and tested according to Class A equipment in GB4824. In the home environment, the equipment may cause radio interference, and protective measures are required.
Basic performance of electromagnetic compatibility claim: when the setting program is the sterilization temperature of 134℃, the maintenance time shall not be less than 3 min. All available space and load during the entire maintenance time shall:
——Should be within the sterilization temperature range;
——The lower limit of the sterilization temperature range is the sterilization temperature, and the upper limit shall not exceed the sterilization temperature + 3℃;
——The difference between the points at the same time shall not exceed 2℃
It is recommended to evaluate the electromagnetic environment before equipment use.
In addition, the use instructions should include the following warnings related to electromagnetic compatibility, such as: " No use of the equipment at strong radiation sources (e. g., unshielded RF sources) that may interfere with the normal operation of the equipment.”
10. Packing List
| No. | Name | Qty. | Remarks |
| 1 | Equipment | 1 | |
| 2 | operating instruction | 1 | |
| 3 | Certificate | 1 | |
| 4 | Pallet | 1 | |
| 5 | Door rubber ring | 1 | |
| 6 | Factory test report | 1 | |
| 7 | Accessories package | 1 | Print Paper (5 volumes) |
Note: Insurance pipe maintenance work should be carried out by professionals, and non-professionals should not replace it by themselves.




